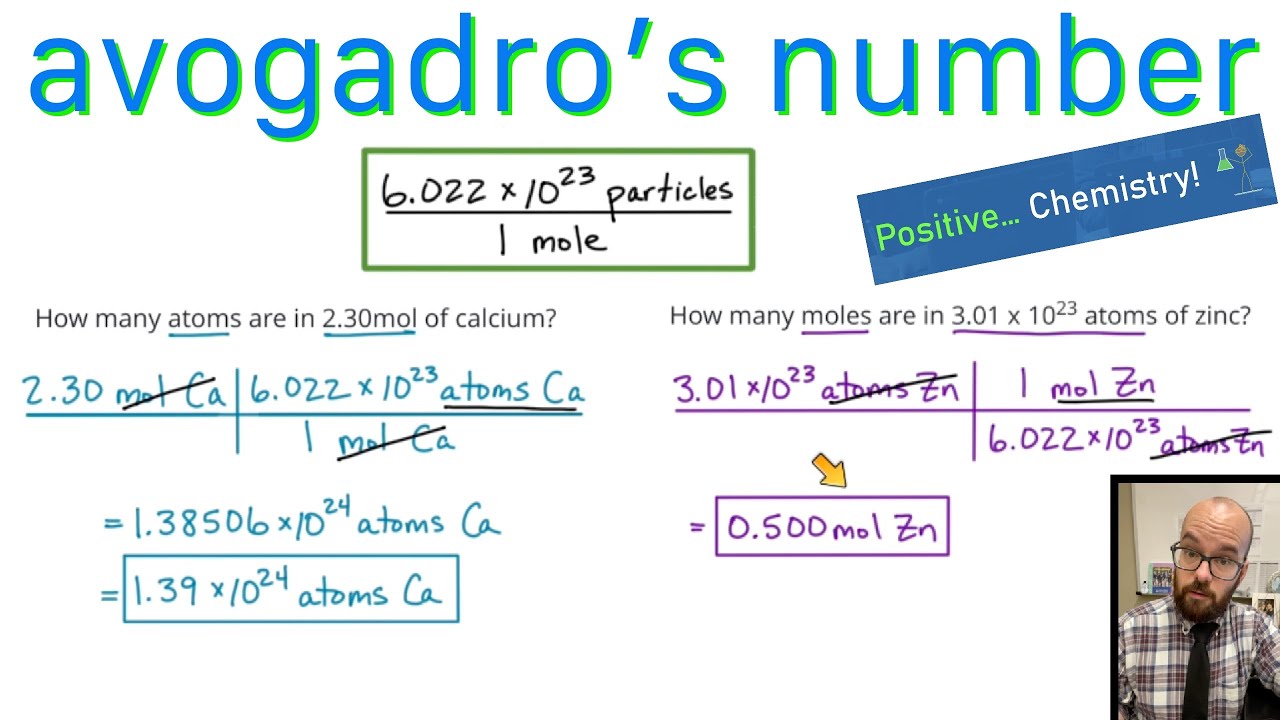
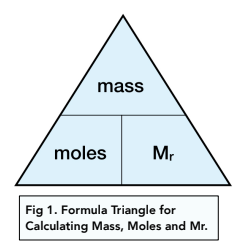
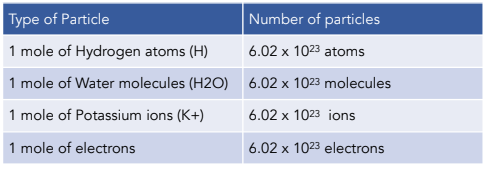
1. Using Avogadro's number, calculate the number of atoms in 0.005 kilograms of carbon. 2. If there are 'x' atoms in 5 grams of carbon, how many atoms are there in 5
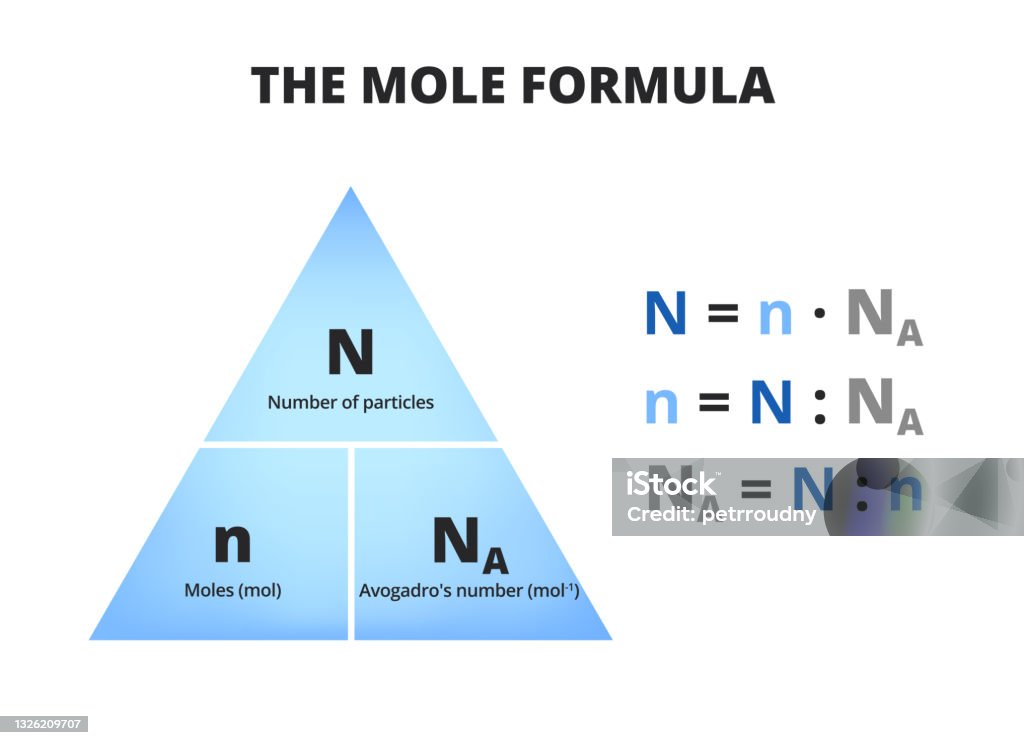
The Mole Formula Triangle Or Pyramid With Avogadro Number Or Avogadro Constant Isolated On White Chemistry Stock Illustration - Download Image Now - iStock

The Mole Concept. Avogadro's Number Avogadro's Number (symbol N) is the number of atoms in grams of carbon. Its numerical value is 6.02 × ppt download

Chemistry - Relation between Mole, Avogadro number and Mass - Atoms and Molecules - Part 8 - YouTube

Lecture 5. THE MOLE Avogadro's number The mole is used when we're talking about numbers of atoms and molecules (tiny particles).moleatomsmolecules The. - ppt download


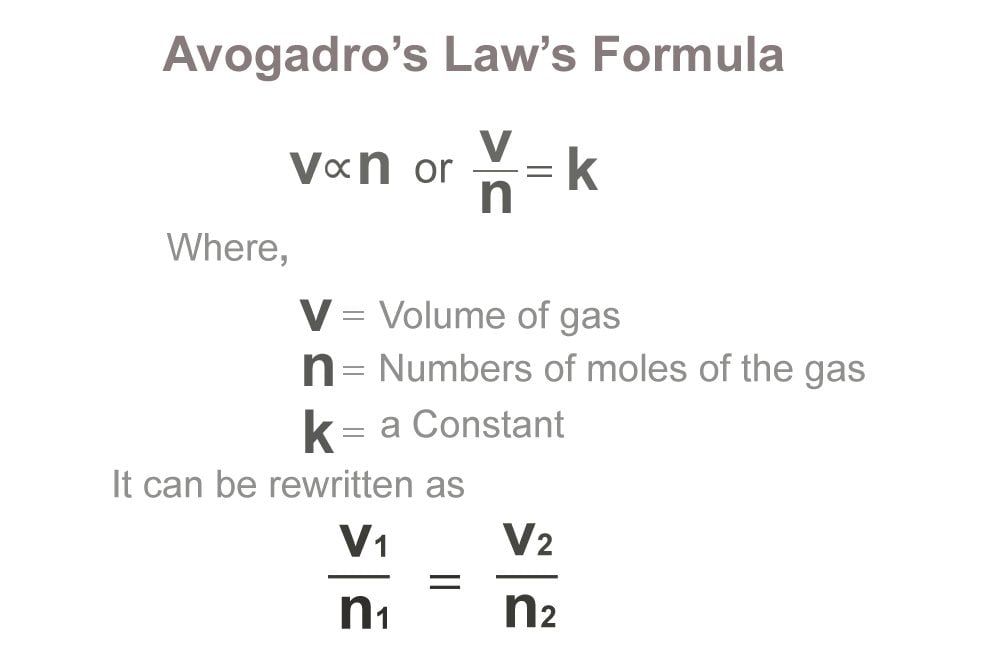
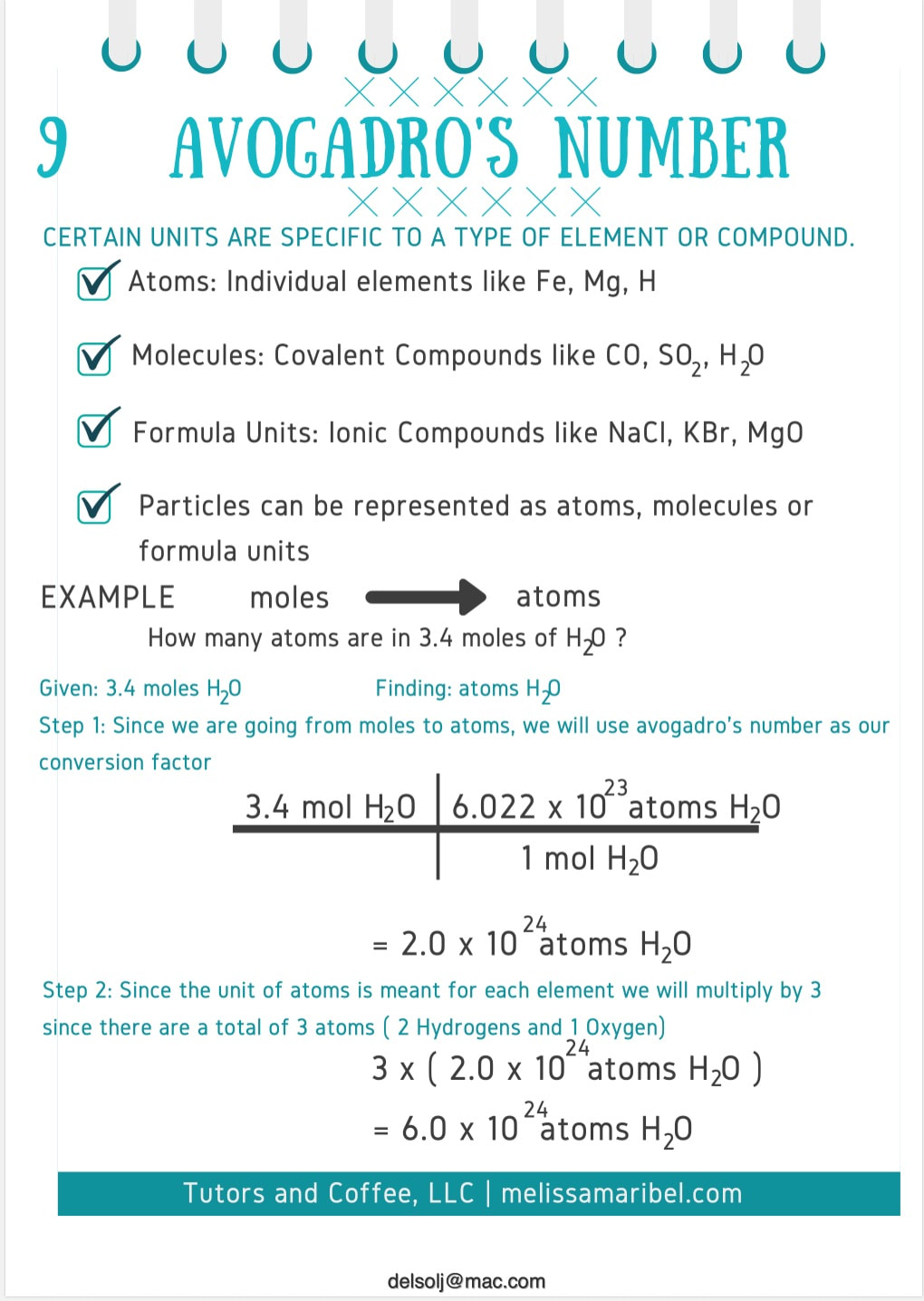


.PNG)